Help prevent bleeds in haemophilia A1,2
ALTUVOCT® is
a first-in-class high-sustained FVIII replacement therapy
indicated
in all age groups
for the
treatment and prophylaxis of bleeding in patients with haemophilia A1
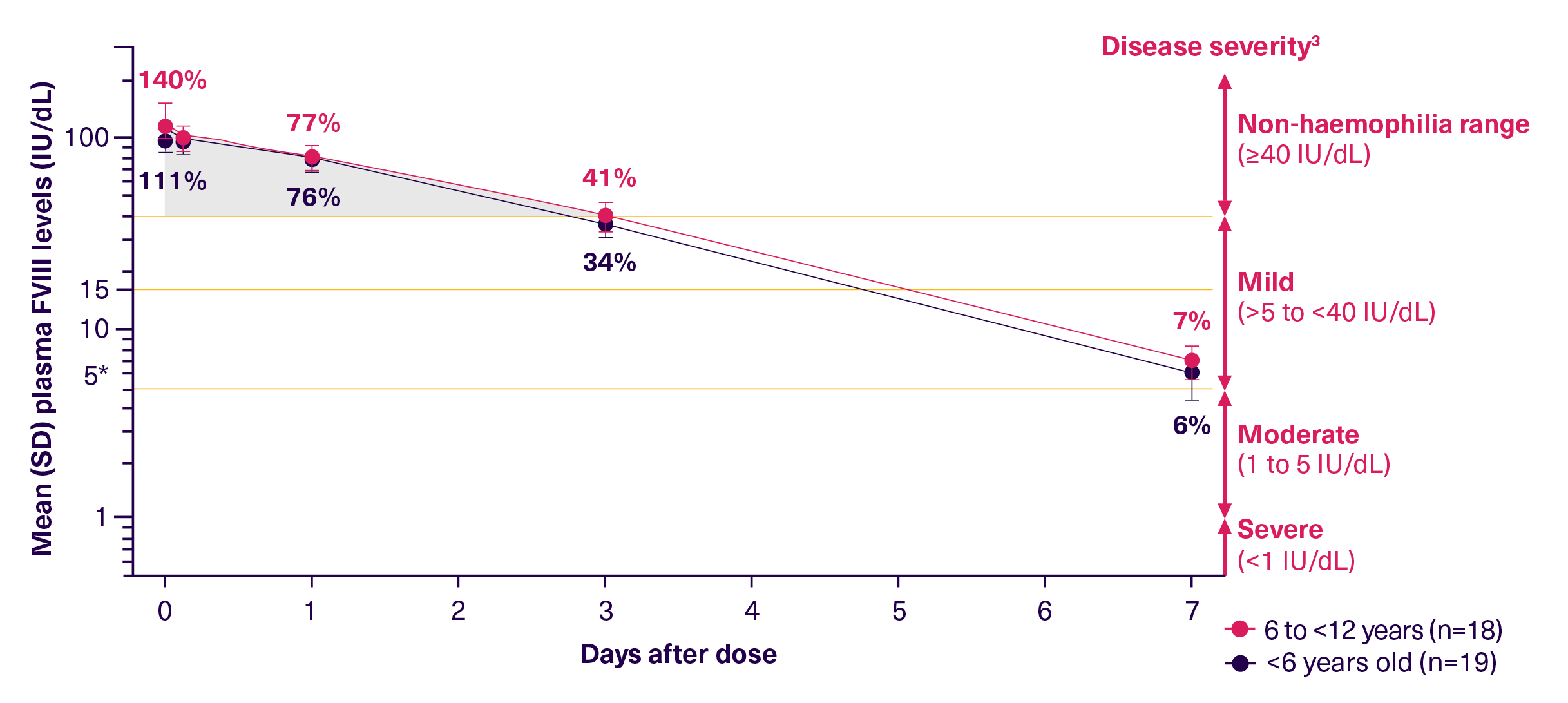
ALTUVOCT® is powered by innovative bioengineering technology that extends its half-life 4x longer than SHL FVIII therapies and 3x longer than EHL FVIII therapies.2-4 As the first and only haemophilia treatment that decouples FVIII from VWF, it provides the opportunity to overcome the VWF-imposed half-life ceiling.2 It can sustain FVIII levels above 40 IU/dL for up to 3 days in children.2 For patients, this means they can stay in the non-haemophilia range for up to 3 days, helping to protect them from bleeds.2,4,5
WITH ONCE-WEEKLY, FIXED-DOSE ALTUVOCT®, YOU CAN:
Sustain
FVIII levels in the non-haemophilia range, FVIII >40 IU/dL, for up to 3 days*2
Harness the physiological benefits of FVIII to its full potential†3 Deliver predictable protection against bleeds†2 Shield patients from joint damage and pain§6 Simplify treatment with once-weekly fixed dosing and the reliability of monotherapy¶1
|
Predictable protection with once-weekly dosing2
In XTEND-Kids, ALTUVOCT® patients achieved zero-to-low bleed rates2
Over 52 weeks of treatment in XTEND-Kids, ALTUVOCT® patients aged <12 years achieved an overall, median ABR of 0 (full analysis set, N=74) with mean ABRs of 0.89 (full analysis set, N=74) and 0.61 (sensitivity analysis set, n=73).2
Could ALTUVOCT® lower bleed rates for your paediatric patients?
|
Over 52 weeks of ALTUVOCT® prophylaxis in XTEND-Kids, most paediatric patients had 0 bleeds2
To prevent arthropathy, prophylaxis must maintain comprehensive protection against joint bleeds.9 In XTEND-Kids, ALTUVOCT® enabled freedom from bleeds for most paediatric patients, with a majority of patients achieving 0 bleeds with a once-weekly fixed dose.2

|
A single treatment across all clinical settings2
ALTUVOCT® supports patients with the reliability of monotherapy across all clinical settings2
When confronted with bleeds or preparing for surgery, the reliability of a monotherapy could offer additional support for patients and caregivers.1
ALTUVOCT® also provides: 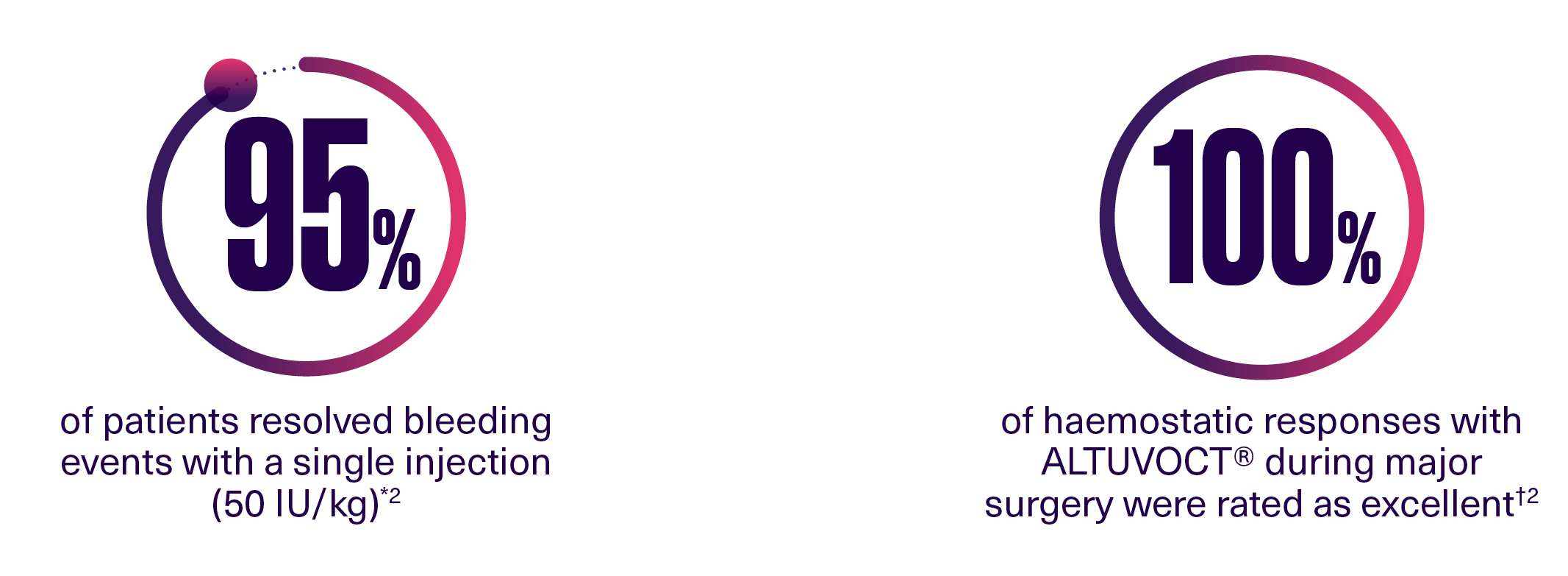
|
ALTUVOCT® has an established safety profile2
ALTUVOCT® was well-tolerated in clinical trials. In XTEND-Kids, there were (N=74):2
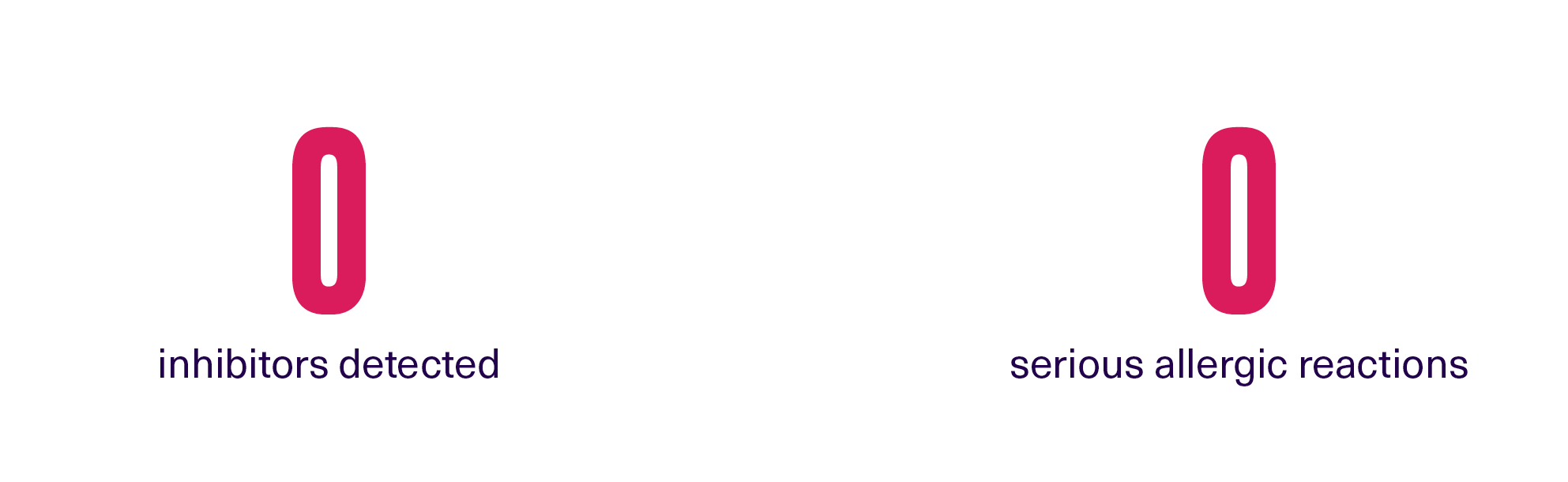
Offer paediatric patients a treatment with proven safety and tolerability |
ABR, annualised bleed rate; AE, adverse event; ED, exposure day; EHL, extended half-life; FVIII, factor VIII; Haem-A-QoL, haemophilia-specific health-related quality of life questionnaire; HJHS, haemophilia joint health score; PROMIS, patient-reported outcome management information system; MoA, mechanism of action; PK, pharmacokinetic; rFVIII, recombinant FVIII; SD, standard deviation; SHL, standard half-life; VWF, Von Willebrand factor.
|