ALTUVOCT® may prevent bleeds in haemophilia A1,2
ALTUVOCT® is
a first-in-class high-sustained FVIII replacement therapy
indicated
in all age groups
for the
treatment and prophylaxis of bleeding in patients with haemophilia A1
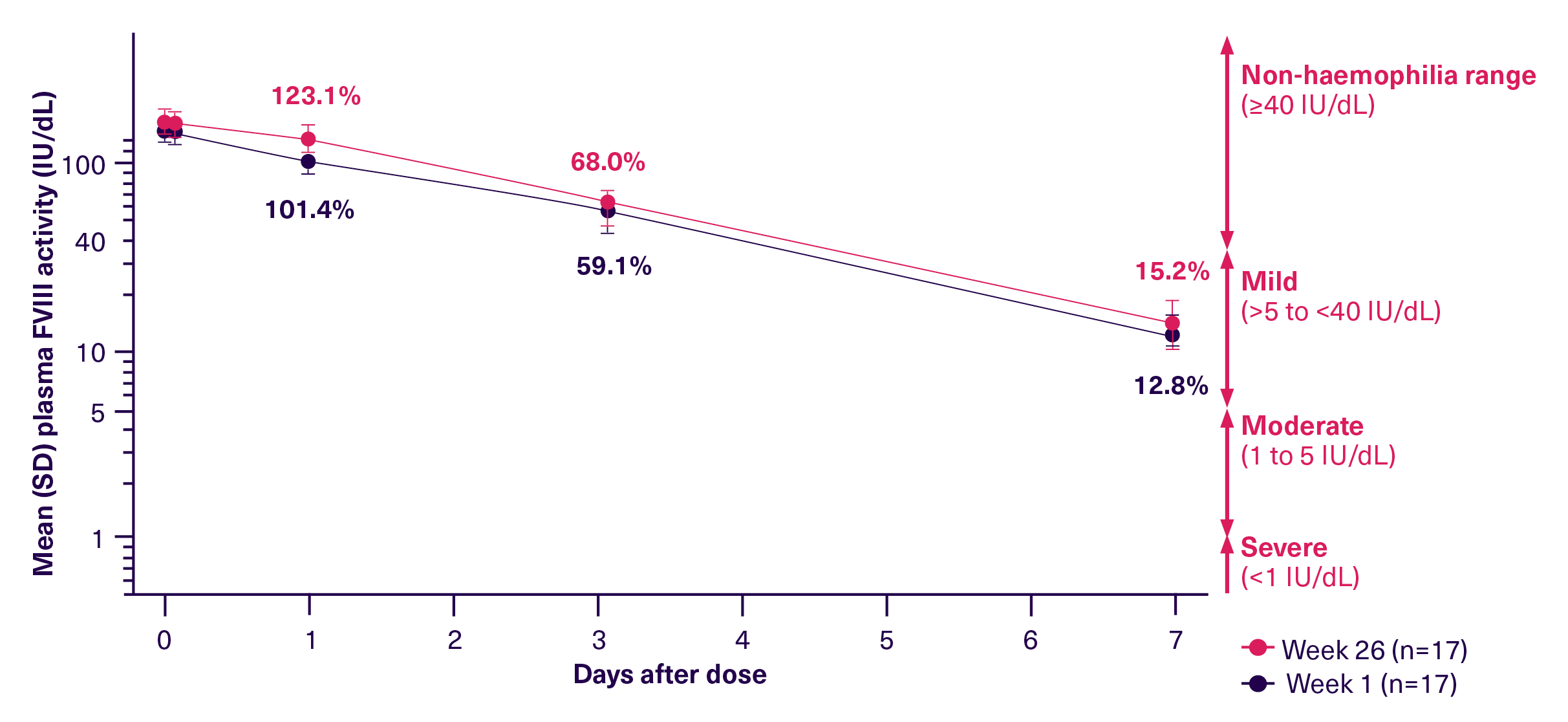
ALTUVOCT® is powered by innovative bioengineering technology, extending its half-life for 4x longer than SHL FVIII therapies and 3x longer than EHL FVIII therapies.2,3 As the first and only approved haemophilia treatment that decouples FVIII from VWF, it provides the opportunity to overcome the VWF-imposed half-life ceiling.2 It can sustain FVIII levels above 40 IU/dL for around 4 days in adults. For patients, this means they can stay in the non-haemophilia range for most of the week, helping to protect them from bleeds.2,4
WITH ONCE-WEEKLY, FIXED-DOSE ALTUVOCT®, YOU CAN:
Sustain
FVIII levels in the non-haemophilia range, FVIII: >40 IU/dL for ~4 days, trough level of 15 IU/dL at day 72,3
Harness the physiological benefits of FVIII to its full potential*2 Deliver predictable protection against bleeds†2 Shield patients from joint damage and pain‡2 Simplify treatment with once-weekly fixed dosing and the reliability of monotherapy§1
|
A single treatment across all clinical settings2
ALTUVOCT® supports patients with the reliability of monotherapy across all clinical settings2
When confronted with bleeds or preparing for surgery, the reliability of a monotherapy could offer additional support.1,2
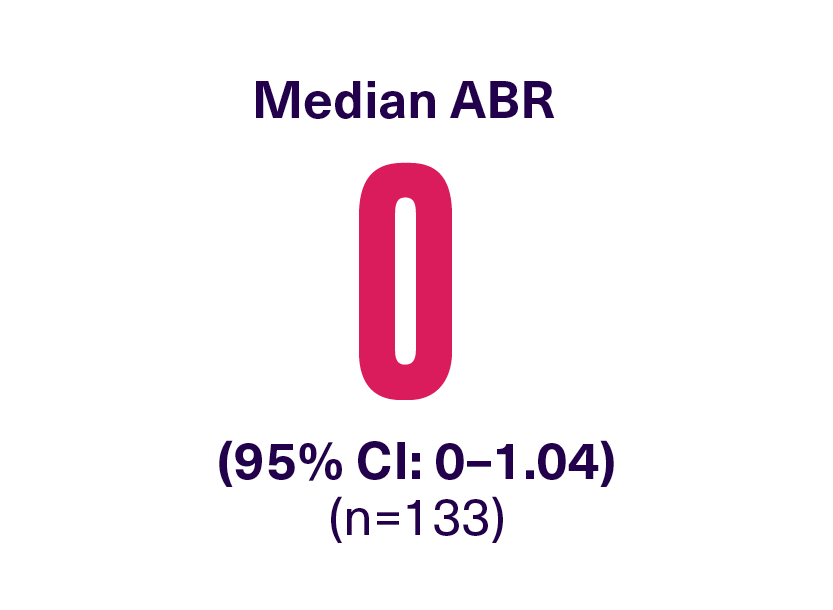
Over 52 weeks in XTEND-1: 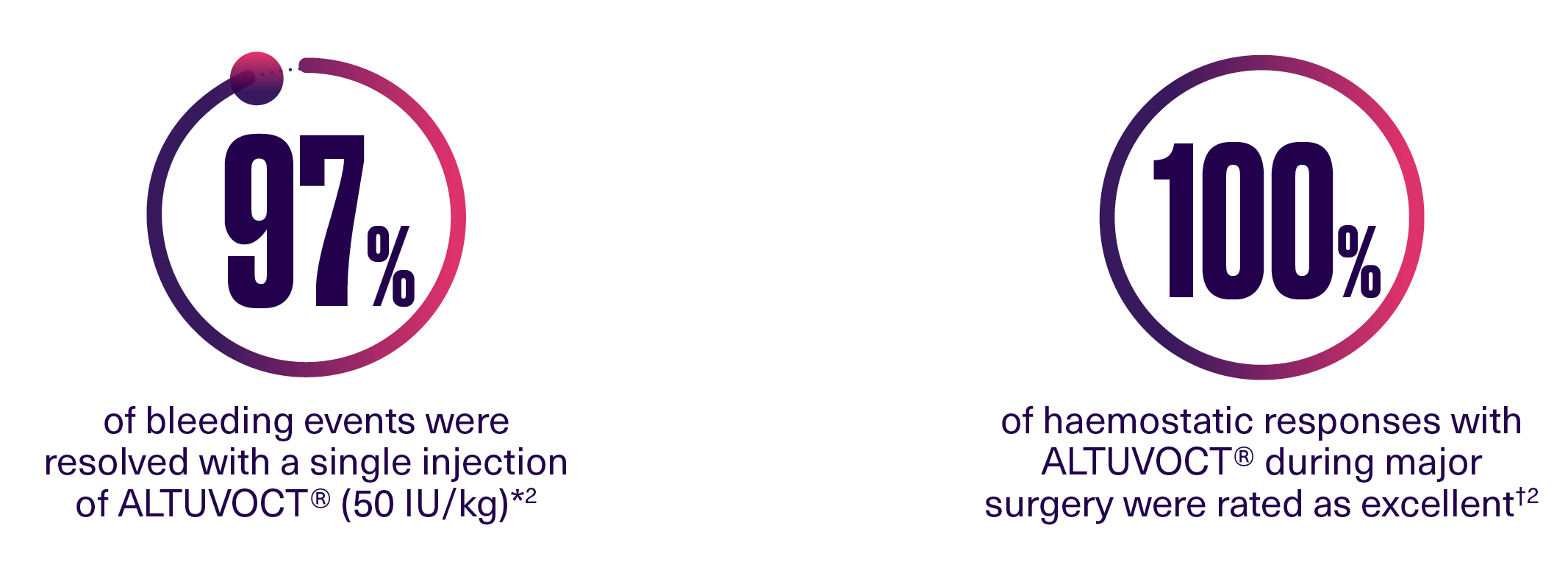
*362 bleeding events occurred during XTEND-1 (n=159), with the majority (74%) in the on-demand treatment period.2
†12 major surgeries were evaluated by the surgeon or investigator (n=11).2 |
ALTUVOCT® has an established safety profile2
ALTUVOCT® was well-tolerated in clinical trials. In XTEND-1, there were (N=159):2
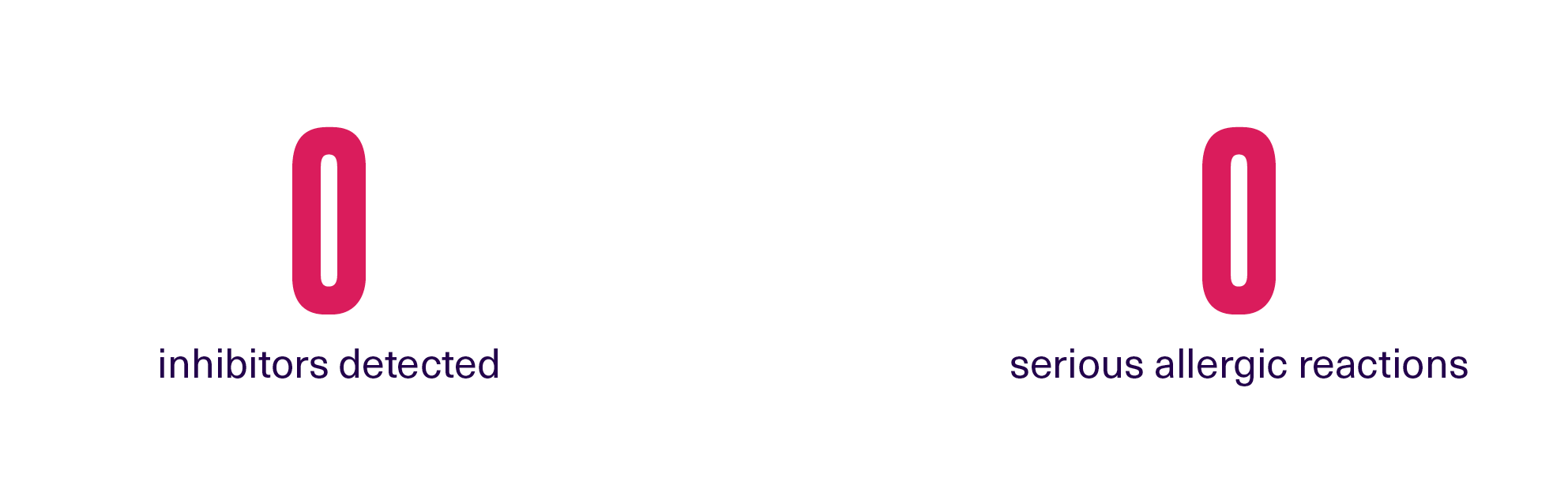
Offer patients a treatment with proven safety and tolerability |
ABR, annualised bleed rate; AE, adverse event; ED, exposure day; EHL, extended half-life; FVIII, factor VIII; Haem-A-QoL, haemophilia-specific health-related quality of life questionnaire; HJHS, haemophilia joint health score; PROMIS, patient-reported outcome management information system; MoA, mechanism of action; PK, pharmacokinetic; rFVIII, recombinant FVIII; SD, standard deviation; SHL, standard half-life; VWF, Von Willebrand factor.
|